|
Сайт "Жизнь вопреки ХПН" создан для образовательных целей, обмена информацией профессионалов в области диализа и трансплантации, информационной и психологической поддержки пациентов с ХПН и их родственников. Медицинские советы врачей могут носить только самый общий характер. Дистанционная диагностика и лечение при современном состоянии сайта невозможны. Советы пациентов медицинскими советами не являются, выражают только их частное мнение, в том числе, возможно, и ошибочное.
Владелец сайта, Алексей Юрьевич Денисов, не несет ответственности за вредные последствия для здоровья людей, наступившие в результате советов третьих лиц, полученных кем-либо на сайте "Жизнь вопреки ХПН"
|
WCN 2009
| |
Vadim
 |
Дата: Пятница, 12.06.2009, 19:54 | Сообщение # 1 |
|
Net-зависимый

Группа: Врач
Сообщений: 1274
Награды: 7
Репутация: 32
Статус:  |
WCN 2009:
Biomarker Allows Early Diagnosis of Acute Kidney Injury
Caroline Helwick Опубликовано на Medscape. June 1, 2009 (Milan, Italy) — Neutrophil gelatinase-associated lipocalin (NGAL) is emerging as a useful early marker of worsening renal function and acute kidney injury (AKI) in a range of clinical situations and patients, according to a number of studies presented here at the World Congress of Nephrology.
Tobias Breidthardt, MD, from the University Hospital, Basel, Switzerland, who presented some of the research on the fluorescence assay, told Medscape Nephrology, "NGAL is all the rage now. It's allowing us to make the diagnosis up to 48 hours before we get serum creatinine results, our traditional method of diagnosis."
NGAL is a small robust protein produced by activated neutrophils in the renal proximal tubules. It is highly inducible during kidney ischemia and appears in urine just 2 to 4 hours after AKI. The current standard test for AKI, serum creatinine (sCr), is not specific for kidney injury and does not accurately detect impaired kidney function until 2 to 3 days after injury. The NGAL assays, which are expected to facilitate diagnosis, are now in the final stages of clinical development.
Useful in Suspected Sepsis
In a large study of 661 patients seen in the emergency department for suspected sepsis, median plasma NGAL concentrations were predictive of renal dysfunction occurring within 72 hours, according to a large multicenter US study reported by Nathan Shapiro, MD, from Beth Israel Deaconess Medical Center in Boston, Massachusetts.
Of the study population, 24 patients (3.6%) developed renal dysfunction (sCr increase > 0.5 mg/dL), and these patients had elevated NGAL concentrations. Median plasma NGAL concentration was 456 ng/mL (interquartile range [IQR], 296 – 727 ng/mL) in patients who developed renal dysfunction compared with 144 ng/mL (IQR, 61 – 302 ng/mL) in those who did not (P < .001).
NGAL was a strong predictor of renal dysfunction, with an area under the curve (AUC) of 0.82 (95% confidence interval [CI], 0.76 – 0.88). By comparison, sCr had an AUC of 0.73 (95% CI, 0.63 – 0.84). Sensitivity for NGAL concentrations higher than 150 ng/mL was 96% (95% CI, 79% – 100%) and specificity was 51% (95% CI, 47% – 55%) for renal dysfunction, Dr. Shapiro reported.
The adjusted odds ratio per quartile for NGAL in a model adjusting for sCr, age, sex, and race was 3.0 (95% CI, 1.6 – 5.8; P < .001), he said.
Predictive After Cardiac Surgery
NGAL's predictive ability was also shown in a prospective international study of 100 patients who underwent cardiac surgery. Investigators evaluated the ability of plasma NGAL, serum cystatin C (another protein that is a measure of kidney function), and a combination of the two to predict the duration and severity of AKI.
NGAL and cystatin C correlated with, and proved to be independent predictors of, the duration and the severity of AKI after surgery, reported Michael Haase, MD, from Charité University Medicine in Berlin, Germany.
Mean AKI duration was 75 ± 42 hours and mean sCr increase was 104 ± 73 µmol/L. On arrival in the intensive care unit (ICU), and 24 hours postoperatively, NGAL and cystatin C each correlated with AKI duration, and the association was stronger when the 2 measures were used together, Dr. Haase said.
"We found that the combination of both renal markers increased their value," Dr. Haase said.
The area under the receiver operating characteristic curve for predicting AKI was 0.85 (95% CI, 0.70 – 0.99) for NGAL combined with cystatin C on arrival in the ICU. Both markers individually and their combination measured on arrival in the ICU also correlated with length of stay in the ICU (P =.037 for NGAL; P = .001 for cystatin C; P < .007 for the combination).
Serial Measures Predictive in Acute Decompensated Heart Failure
In patients with acute decompensated heart failure (ADHF), serial measurements of NGAL were useful in identifying worsening renal function, according to researchers from the University Hospital in Basel, Switzerland.
Their prospective study included 50 consecutive patients presenting to the emergency department with ADHF. Plasma NGAL samples were collected upon admission and at 6 hours. Serum creatinine was evaluated at baseline and daily for 4 days.
"We wanted to see if NGAL, which has performed well in other kidney areas, would be useful in ADHF too, since there is often a lot of 'background' noise in this condition," Dr. Breidthardt said.
Worsening renal function (sCr increase ≥ 0.3 mg/dL compared with baseline) occurred in 13 cases (26%). In these 13 cases, sCr increases were first observed at 24 hours in 3 cases, at 48 hours in 3 cases, and after 96 hours in 7 cases. The median baseline NGAL concentration in the entire population was 95 ng/mL (IQR, 60 – 200 ng/mL) and the median 6-hour NGAL concentration was 81 ng/mL (IQR, 64 – 177 ng/mL), Dr. Breidthardt reported.
The ratio NGAL level (ratio of 6-hour NGAL concentration to baseline) was statistically significant for predicting worsening renal failure (P < .05). The median was 0.88 (IQR, 0.65 – 1.10) in patients without worsening renal function compared with 1.22 (IQR, 0.96 – 1.38) in patients with worsening renal function.
At a cut-off value of 1.20 ng/mL (ie, a 20% increase over baseline), the odds ratio for worsening renal failure was 4.23 (95% CI, 1.10 – 16.2), the study found.
"Taking only one spot measurement on admission was not useful, but 2 serial measurements were predictive," Dr. Breidthardt added. "It's possible the prognostic value can be improved further using additional NGAL monitoring throughout the patient's hospitalization."
Chirag Parikh, MD, PhD, associate professor of medicine at Yale University Medical School in New Haven, Connecticut, in a separate session applauded the development of new methods for assessing renal injury, which will allow for more rapid treatment and thus could help reduce the cost of treating AKI, now estimated to be $10 billion a year. "Serum creatinine testing is greatly responsible for the lack of progress in the field of kidney injury. It is a nonspecific marker that delays the diagnosis of AKI," he commented.
"Earlier diagnosis of evolving AKI could result in quicker changes in patient management to make potentially life-saving therapeutic interventions and also to stop harmful interventions," added Patrick Murray, MD, professor at University College Dublin and Mater Misericordiae University Hospital in Dublin, Ireland. "More accurate differential diagnosis of AKI could direct appropriate therapy, and more accurate, serial staging could help us in making prognostic stratifications and assessing the current and future severity of injury," he explained.
These studies were funded by Biosite Diagnostics, Abbott Diagnostics, and Inverness Medical. Dr. Haase has received travel expenses from Biosite Incorporated and Abbott Diagnostics. World Congress of Nephrology 2009:
A Joint Meeting of the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) and the International Society of Nephrology (ISN): Abstracts M196, M187, and M180. Presented May 25, 2009. Смотри также......
Festina lente
Сообщение отредактировал Vadim - Пятница, 12.06.2009, 21:53 |
| |
| |
Vadim
 |
Дата: Пятница, 12.06.2009, 21:28 | Сообщение # 2 |
|
Net-зависимый

Группа: Врач
Сообщений: 1274
Награды: 7
Репутация: 32
Статус:  |
WCN 2009:
New Class of Agents May Improve Renal Function in Diabetic Nephropathy
Caroline Helwick June 1, 2009 (Milan, Italy) — Pirfenidone, a novel oral antifibrotic agent, significantly improves renal function in patients with diabetic nephropathy, compared with placebo, according to results from a randomized pilot study presented at a late-breaking clinical-trials session here at the World Congress of Nephrology, a Joint Meeting of the European Renal Association–European Dialysis and Transplant Association and the International Society of Nephrology. "Diabetes is the number 1 cause of renal failure in the United States. We urgently need novel therapies for progressive diabetic nephropathy, since our existing therapies — such as ACE inhibitors and angiotensin-receptor blockers [ARBs] — are not completely effective in arresting progression, especially at more advanced stages of disease," said lead author Kumar Sharma, MD, professor of medicine and director of renal translational medicine at the University of California School of Medicine, San Diego. Decline in glomerular filtration rate (GFR) is mediated by glomerular matrix accumulation. Pirfenidone acts against connective tissue growth factor and has shown antifibrotic effects in focal glomerular sclerosis and idiopathic pulmonary fibrosis. In Japan, pirfenidone is now approved for the treatment of the latter. Based on preclinical and open-label clinical studies, Dr. Sharma and colleagues initiated a randomized double-blind placebo-controlled trial of pirfenidone in patients with diabetic nephropathy and impaired estimated (e)GFR to assess the change in eGFR after 1 year of taking the study drug. Enrollment criteria included impaired eGFR (<80 mL/min per 1.73 m2) on existing therapy with ACE inhibitors, ARBs, or the combination, and a clinical diagnosis of diabetic nephropathy. The primary end point was change in eGFR from baseline to the end of the 1-year study. Investigators randomized 77 patients to placebo, low-dose pirfenidone (1200 mg/d), or high-dose pirfenidone (2400 mg/d). Because of a high number of protocol violations, drop-outs due to adverse events, and other reasons for lack of follow-up, the study was completed by 50 patients, which forms the basis of the preliminary analysis. For the primary end point, mean change in eGFR at 1 year, pirfenidone 1200 mg/day was associated with significantly greater benefit than placebo or the higher active dose. Mean eGFR change from baseline was +3.09 ± 2.65 mL/min per 1.73 m2 with pirfenidone 1200 mg/day and –2.53 ± 1.29 mL/min per 1.73 m2 with placebo (P = .046), Dr. Sharma reported. Mean eGFR change for the high-dose group fell between the other 2 groups, at –1.74 ± 2.40 mL/min per 1.73 m2 (P = .756 vs placebo). "The improvement in eGFR in the [low-dose] group was not due to a reduction of albuminuria or to good glycemic control," Dr. Sharma added. "There was no protective effect on the urinary albumin-creatinine ratio." Fourteen patients did not complete the study because of adverse events (4 in the placebo group, 5 in the low-dose group, and 5 in the high-dose group). Of the initial 77 patients, 5 initiated hemodialysis after enrolling in the study (4 in the placebo group and 1 in the high-dose group). Adverse events were similar among the groups. The most common symptoms were gastrointestinal upset and fatigue. "Based on this randomized double-blind placebo-controlled pilot study, we believe that oral antifibrotic therapy with pirfenidone at 1200 mg/day for 1 year holds promise for stabilizing renal function in patients with diabetic nephropathy," Dr. Sharma concluded. "Larger phase 2 and 3 trials are indicated to prove its efficacy in progressive kidney disease." Commenting on the study, Richard Glassock, MD, professor emeritus at the David Geffen School of Medicine, University of California, Los Angeles, said he is concerned that antifibrotic agents could have a negative effect in patients with diabetic nephropathy. "I am concerned about the safety of these antifibrotic agents in such patients," he said. "In the course of planning the pivotal phase 3 studies, the investigators might consider the potential effects on the vulnerable plaque in patients with type 2 diabetes, because the fibrous envelope that eventually encompasses the lipid-rich plaque has importance in preventing thrombosis of the coronary arteries. "I hope the investigators will characterize the risk for myocardial infarction in the study population," Dr. Glassock added, "and perhaps include some exclusion criteria that will prevent this drug from getting a bad reputation early on." The primary supporter of the study was the National Institutes of Health. InterMune Inc provided the study drug and supplemental funds for this clinical study, but the company was not involved in data analysis or in the writing of the abstract. The authors have disclosed no relevant financial relationships. World Congress of Nephrology 2009: A Joint Meeting of the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) and the International Society of Nephrology (ISN): Abstract Sa775. Presented May 25, 2009.
Festina lente
Сообщение отредактировал Vadim - Пятница, 12.06.2009, 21:29 |
| |
| |
Vadim
 |
Дата: Пятница, 12.06.2009, 21:39 | Сообщение # 3 |
|
Net-зависимый

Группа: Врач
Сообщений: 1274
Награды: 7
Репутация: 32
Статус:  |
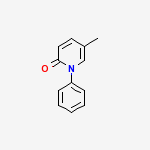
Название по номенклатуре Междунаро́дного сою́за чи́стой и прикладно́й хи́мии : 5-methyl-1-phenylpyridin-2-one | CAS Registry Number: 53179-13-8 Синонимы: Deskar, PIRFENIDONE, Pirespa, Pirfenidone (Deskar), Pirfenidone [USAN:INN], Tocris-1093, Pirfenidonum [INN-Latin], Pirfenidona [INN-Spanish], AMR-69, Lopac-P-2116, AMR 69, Lopac0_000907, MLS000860042, P2116_SIGMA, Pirfenidone (JAN/USAN/INN), C12H11NO, 5-Methyl-1-phenyl-2-(1H)-pyridone, 5-Methyl-1-phenyl-2(1H)-pyridone, Bio1_000397, Bio1_000886
Молекулярная формула: C12H11NO Молекулярный вес: 185.221840 [г/моль] А также:Pharmacokinetics of an antifibrotic agent, pirfenidone, in haemodialysis patients
И еще: Neutrophil Gelatinase-Associated Lipocalin (NGAL) and Progression of Chronic Kidney Disease
Festina lente
Сообщение отредактировал Vadim - Пятница, 12.06.2009, 21:59 |
| |
| |
|
 Участники
Участники Правила форума
Правила форума Поиск
Поиск









